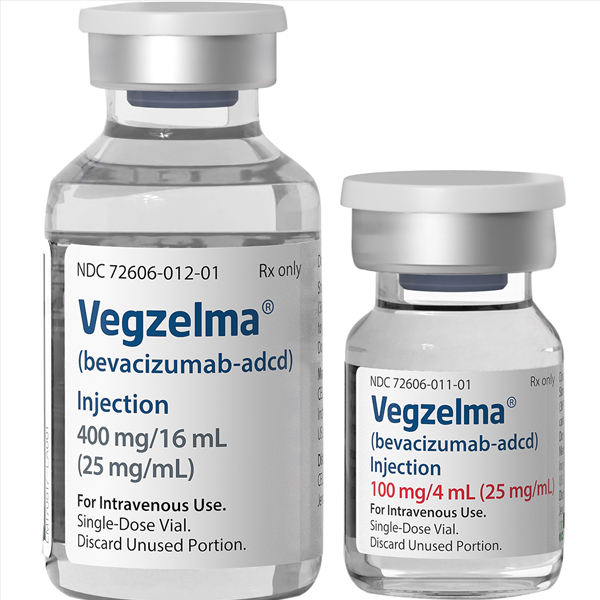
Buy Vegzelma (Bevacizumab-adcd)
$375.00 – $1,125.00
Vegzelma is indicated for the treatment of six types of cancer: metastatic colorectal cancer; recurrent or metastatic non-squamous non-small cell lung cancer (nsNSCLC); recurrent glioblastoma; metastatic renal cell carcinoma; persistent, recurrent, or metastatic cervical cancer; and epithelial ovarian, fallopian tube, or primary peritoneal cancer.
Description
What is Vegzelma (bevacizumab-adcd)?
On September 28, the U.S. Food and Drug Administration (FDA) approved bevacizumab-adcd (Vegzelma), a biosimilar to bevacizumab (Avastin), for the treatment of six types of cancer: metastatic colorectal cancer; recurrent or metastatic nonsquamous non–small cell lung cancer (NSCLC); recurrent glioblastoma; metastatic renal cell carcinoma; persistent, recurrent, or metastatic cervical cancer; and epithelial ovarian, fallopian tube, or primary peritoneal cancer.
Where has Vegzelma (bevacizumab-adcd) been approved?
On September 28, the U.S. Food and Drug Administration (FDA) approved bevacizumab-adcd (Vegzelma), a biosimilar to bevacizumab (Avastin), for the treatment of six types of cancer: metastatic colorectal cancer; recurrent or metastatic nonsquamous non–small cell lung cancer (NSCLC); recurrent glioblastoma; metastatic renal cell carcinoma; persistent, recurrent, or metastatic cervical cancer; and epithelial ovarian, fallopian tube, or primary peritoneal cancer.
The European Medicines Agency decided that, in accordance with EU requirements for biosimilar medicines, Vegzelma has a highly similar structure, purity and biological activity to Avastin (an already EMA approved medicine) and is distributed in the body in the same way. The data were considered sufficient to conclude that Vegzelma will behave in the same way as Avastin in terms of effectiveness and safety in its authorised uses. Therefore, the Agency’s view was that, as for Avastin, the benefits of Vegzelma outweigh the identified risks and it can be authorised for use in the EU.
Additional information
| Package | 100 mg/4 mL (25 mg/mL), 400 mg/16 mL (25 mg/mL) |
|---|


Sarah Martinez –
Vegzelma has been a transformative addition to my treatment regimen for Crohn’s disease. Its targeted approach to modulating the immune response has significantly reduced my symptoms and improved my overall quality of life. Unlike previous medications, Vegzelma has provided relief without causing debilitating side effects. I appreciate the convenience of its once-daily oral dosage, making it easy to integrate into my routine. Since starting Vegzelma, I’ve experienced fewer flare-ups and less discomfort, allowing me to focus on living a fuller, more active life. The consistent support and guidance from my healthcare team have been invaluable in optimizing my treatment plan with Vegzelma. Overall, I’m grateful for the positive impact this medication has had on managing my condition, and I would highly recommend it to others seeking effective relief from Crohn’s disease symptoms