Buy Darzalex (daratumumab)
$395.45 – $1,185.25
Darzalex (daratumumab) is a human CD38-directed monoclonal antibody used to treat multiple myeloma
Who is Darzalex (daratumumab) for?
Darzalex (daratumumab) is indicated for patients with:
-
newly diagnosed multiple myeloma who are ineligible for autologous stem cell transplant (first-line treatment). For this indication, it is used in combination with bortezomib, melphalan, and prednisone.
-
multiple myeloma who have received at least one prior therapy (second-line treatment). For this indication, it is used in combination with lenalidomide and dexamethasone, or bortezomib and dexamethasone.
-
multiple myeloma who have received at least two prior therapies including lenalidomide and a proteasome inhibitor (third-line treatment). For this indication, it is used in combination with pomalidomide and dexamethasone.
-
multiple myeloma who have received at least three prior lines of therapy including a proteasome inhibitor (PI) and an immunomodulatory agent or who are double-refractory to a PI and an immunomodulatory agent (fourth-line treatment). For this indication, it is used as monotherapy.
Description
How does Darzalex (daratumumab) work?
Multiple myeloma is a form of blood cancer that occurs in infection-fighting plasma cells (a type of white blood cell) found in the bone marrow. These cancerous cells multiply, producing an abnormal protein and pushing out other healthy blood cells from the bone marrow.
Daratumumab is a monoclonal antibody that works by helping certain cells in the immune system attack these cancer cells. One of the antigens expressed on the surface of multiple myeloma cells is called CD38. Anti-CD38 antibodies, like daratumumab, target multiple myeloma cells by binding to the CD38 antigen and then signaling the patient’s immune system to attack the tumour.
Where has Darzalex (daratumumab) been approved?
Darzalex (daratumumab) was approved for multiple myeloma by:
- Food and Drug Administration (FDA), USA:
- May 7, 2018, as first-line therapy in combination with bortezomib, melphalan, and prednisone.
- November 21, 2016, as second-line therapy in combination with lenalidomide and dexamethasone, or bortezomib and dexamethasone.
- June 16, 2017, as third-line therapy in combination with pomalidomide and dexamethasone.
- November 16, 2015, as fourth-line therapy.
- EMA (EU) on May 20, 2016.
- Health Canada on June 29, 2016.
- TGA (AUS) on July 17, 2017
- Medsafe on November 20, 2017.
How is Darzalex (daratumumab) taken?
The recommended dose is 16 mg/kg body weight according to the following schedule.
As monotherapy and in combination with lenalidomide and low-dose dexamethasone:
-
weekly infusions for week 1–8 (total of 8 infusions)
-
from week 9–24 every 2 weeks (total of 8 infusions)
-
from week 25 onwards until disease progression every four weeks.
In combination with bortezomib and dexamethasone:
-
weekly infusions for week 1–9 (total of 9 infusions)
-
from week 10–24 every 3 weeks (total of 5 infusions)
-
from week 25 onwards until disease progression every four weeks.
In combination with bortezomib, melphalan, and prednisone (6-week cycle regimen):
-
weekly infusions for week 1–6 (total of 6 infusions)
-
from week 7–54 every 3 weeks (total of 16 infusions)
-
from week 55 onwards until disease progression every four weeks
Complete information about daratumumab dosage and administration can be found in the resources section.
Note: Consult your treating doctor for personalised dosing.
Are there any known adverse reactions or side effects of Darzalex (daratumumab)?
Common adverse reactions
The most common side effects adverse reactions (incidence ≥20%) listed in the prescribing information include1:
- infusion reactions
- neutropenia (low white blood cells count)
- thrombocytopenia (low blood platelet count)
- fatigue (tiredness)
- nausea (feeling sick)
- diarrhea
- vomiting
- muscle spasms
- back pain
- pyrexia (fever)
- cough
- dyspnea (Shortness of breath)
- dizziness (feeling of being lightheaded, woozy, or unbalanced)
- insomnia
- peripheral edema (accumulation of fluids in the limbs)
- peripheral sensory neuropathy
- upper respiratory tract infection.
Serious adverse reactions
The serious adverse reactions listed in the prescribing information include:
- infusion reactions
- neutropenia
- thrombocytopenia.
Additional information
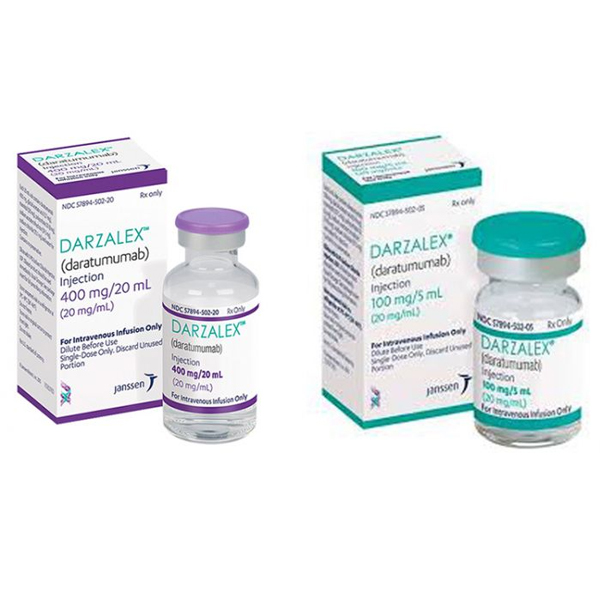
| Vial | 100 mg/5 mL, 400 mg/20 mL |
|---|


Jennifer Peterson –
Darzalex has been a beacon of hope in my battle against multiple myeloma. When conventional treatments failed to curb the progression of my disease, Darzalex stepped in as a game-changer. Its innovative mechanism of action, targeting CD38-positive cells, has provided me with tangible results that I once thought were unattainable. While I’ve encountered some mild side effects like fatigue and infusion reactions, they pale in comparison to the prospect of remission. Darzalex has not only halted the advance of my cancer but has also given me the chance to envision a future beyond the confines of my diagnosis. It’s more than just a medication; it’s a testament to the power of scientific advancement and perseverance. I am immensely grateful to the researchers and medical professionals who have brought Darzalex into existence, guiding me along a path illuminated with hope and possibility.